✔ 100% Authentic Product
👁️ Currently Viewing 2072
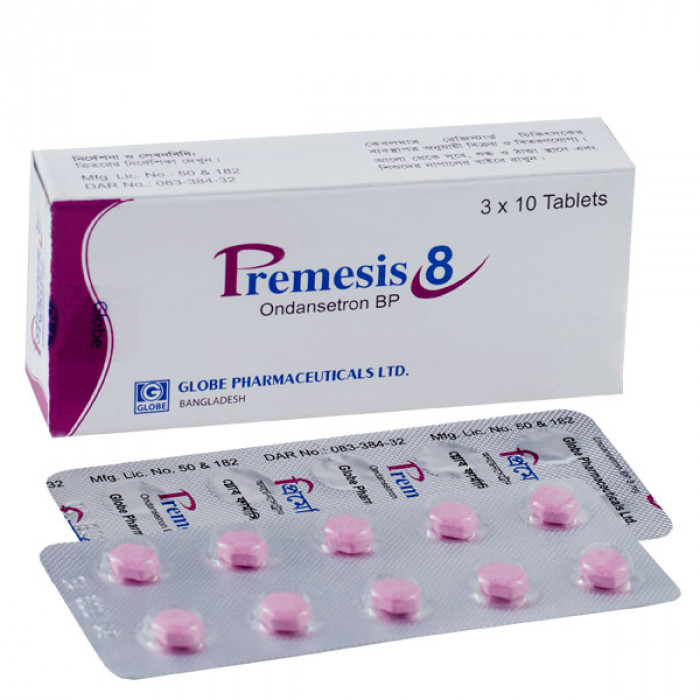
Premesis 8mg 10pcs
Generic Name: Ondansetron
Manufacturer: Globe Pharmaceuticals Ltd.
Discount
Price: ৳ 10
MRP:
৳
10
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Premesis is an antiemetic medicine commonly used to control nausea and vomiting due to certain medical conditions like stomach upset. It is also used to prevent nausea and vomiting caused due to any surgery, cancer drug therapy or radiotherapy. Premesis may be used alone or with other medications and can be taken with or without food. Your doctor will suggest the appropriate dose depending on what you are taking it for. The first dose is normally taken before the start of surgery, chemotherapy or radiotherapy. After these treatments, take any further doses as prescribed by your doctor (normally only for a few days at most). Take it regularly at the same time(s) each day to get the most benefit. Be careful not to take too much. This medicine does not relieve other side effects associated with cancer treatments. Also, it has little effect on vomiting caused by motion sickness. The most common side effects of taking this medicine include headache, diarrhea or constipation and feeling tired. These symptoms should disappear when you stop taking the medicine. However, if these side effects bother you or do not go away, your doctor may be able to suggest ways of preventing or reducing them. Before taking this medicine, tell your doctor if you have heart or liver problems or a blockage in your stomach or intestines. Also, tell your doctor about any other medicines you might be taking, especially medicines to treat epilepsy, heart problems, cancer and depression. These may affect, or be affected by, this medicine. If you are pregnant or breastfeeding, ask for advice from your doctor.
Uses of Premesis
Nausea
Vomiting
Side effects of Premesis
Common
Constipation
Diarrhea
Fatigue
Headache
How to use Premesis
Take this medicine in the dose and duration as advised by your doctor. Do not handle the tablets with wet hands. Put it in your mouth but do not swallow it. Premesis may be taken with or without food, but it is better to take it at a fixed time.
How Premesis works
Premesis is an antiemetic medication. It works by blocking the action of a chemical messenger (serotonin) in the brain that may cause nausea and vomiting during anti-cancer treatment (chemotherapy) or after surgery.
What if you forget to take Premesis?
If you miss a dose of Premesis, take it as soon as possible. However, if it is almost time for your next dose, skip the missed dose and go back to your regular schedule. Do not double the dose.
Quick Tips
You have been prescribed Ondace 4mg Tablet MD for the prevention of nausea and vomiting caused after surgery or due to chemotherapy and radiotherapy.
Unlike some other nausea medicines, the side effects of Ondace 4mg Tablet MD are relatively mild.
It is fast-acting and starts working within 30 minutes.
If you vomit within one hour of taking a dose, take another dose.
Avoid heavy meals and try eating small nourishing snacks throughout the day. Also, sip water regularly to help avoid dehydration.
Brief Description
Indication
Prevention of nausea-vomiting associated with chemotherapy, Prevention of nausea & vomiting associated with radiotherapy, Prevention of post-operative nausea & vomiting, Nausea-vomiting in gastroenteritis, Nausea vomiting in pregnancy
Administration
May be taken with or without food. Reconstitution: Prior to IV infusion, dilute in 50 mL dextrose 5% inj or normal saline.
Adult Dose
Oral Adult: Prevention of nausea & vomiting associated with chemotherapy: Highly emetogenic chemotherapy: 24 mg once 30 minutes before start of single-day chemotherapy. Moderately emetogenic chemotherapy: 8 mg (one 8 mg tablet) administered 30 minutes before start of emetogenic chemotherapy. A further 8 mg dose should be administered after 8 hours of the first dose. One 8 mg tablet should be administered twice a day (every 12 hours) for 1-2 days after completion of chemotherapy. Prevention of nausea & vomiting associated with radiotherapy: Total body irradiation: 8 mg 1-2 hours before each fraction of radiotherapy administered each day. Single high-dose fraction radiotherapy to abdomen: 8 mg 1-2 hours before radiotherapy, then 8 mg every 8 hours after 1st dose for 1-2 days after radiation completed. Daily fractionated radiotherapy to abdomen: 8 mg 1-2 hours before radiotherapy, then 8 mg every 8 hours after 1st dose for each day of radiotherapy. Prevention of post-operative nausea & vomiting: 16 mg 1 hour before induction of anesthesia. Nausea-vomiting in gastroenteritis Adult: 8 mg three times daily. Nausea vomiting in pregnancy 8 mg 2-3 times daily Parenteral Prevention of nausea-vomiting associated with chemotherapy 0.15 mg/kg IV over 15 min administered 30 min before chemotherapy, then 4 and 8 hr after first dose; not to exceed 16 mg Prevention of post-operative nausea-vomiting 4 mg IV/IM immediately before anesthesia or after procedure. The rate of administration should not be less than 30 seconds, preferably over 2 to 5 minutes. Rectal Nausea and vomiting associated with cancer chemotherapy Adult: As suppository: 16 mg given 1-2 hr prior to treatment. Elderly: No dosage adjustment needed. Severe hepatic impairment (Child-Pugh score ?10): Not to exceed 8 mg/day
Child Dose
Oral Moderate emetogenic cancer chemotherapy: Child (4-11 years): 4 mg tablet should be taken 30 minutes before the start of chemotherapy. The other 2 doses should be taken 4 and 8 hours after the first dose. Then 4 mg tablet should be administered 3 times a day (every 8 hours) for 1-2 days after completion of chemotherapy. Prevention of nausea & vomiting associated with radiotherapy: Child >12 years Total body irradiation: 8 mg 1-2 hours before each fraction of radiotherapy administered each day. Single high-dose fraction radiotherapy to abdomen: 8 mg 1-2 hours before radiotherapy, then 8 mg every 8 hours after 1st dose for 1-2 days after radiation completed. Daily fractionated radiotherapy to abdomen: 8 mg 1-2 hours before radiotherapy, then 8 mg every 8 hours after 1st dose for each day of radiotherapy. Nausea-vomiting in gastroenteritis Children (>1 month): 0.15 mg/kg body weight three times daily. Prevention of post-operative nausea-vomiting Child >12 years: 16 mg 1 hour before induction of anesthesia. Parenteral Prevention of nausea-vomiting associated with chemotherapy Child>6 months: 0.15 mg/kg IV over 15 min administered 30 min before chemotherapy, then repeated 4 and 8 hr after first dose; not to exceed 16 mg/dose Prevention of post-operative nausea-vomiting Child: 1 month-12 yrs (<40 kg): 0.1 mg/kg; 40 kg: 4 mg. Give IV as single dose immediately before induction of anesthesia; or shortly post-op if nausea or vomiting occurs. The rate of administration should not be less than 30 seconds, preferably over 2 to 5 minutes.
Renal Dose
Renal impairment: Dose adjustment not necessary
Contraindication
Use with apomorphine (profound hypotension).
Mode of Action
Ondansetron antagonises selective 5-HT3-receptor, blocking serotonin, both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone. This action of ondansetron gives it its antiemetic property to prevent emesis due to acute chemotherapy mediated by serotonin.
Precaution
May mask progressive ileus and/or gastric distension. Severe hepatic impairment. Pregnancy and lactation. Monitoring Parameters ECG monitoring of patients w/ electrolytes abnormalities e.g. hypomagnesaemia or hypokalaemia, CHF or bradyarrhythmias and on medication that can prolong QT interval.
Side Effect
>10% Headache (9-27%),Malaise/fatigue (9-13%),Constipation (6-11%) 1-10% Hypoxia (9%),Drowsiness (8%),Diarrhea (2-7%),Dizziness (7%),Fever (2-8%),Gynecologic disorder (7%),Anxiety (6%),Urinary retention (5%),Pruritus (2-5%),Injection-site pain (4%),Paresthesia (2%),Cold sensation (2%),Elevated liver function test results (1-5%) <1% Cardiac: Arrhythmias (including ventricular and supraventricular tachycardia, premature ventricular contractions, and atrial fibrillation), bradycardia, electrocardiographic alterations (including second-degree heart block, QT/QTc interval prolongation, and ST segment depression), palpitations, and syncope; rarely and predominantly with intravenous ondansetron, transient ECG changes including QT/QTc interval prolongation have been reported Gastrointestinal: Nausea and vomiting Anaphylaxis ECG alterations: Arrhythmias; prolongation of PR, QRS, and QT intervals Hepatobiliary: Specific hepatic enzyme abnormalities, hepatic necrosis, and abnormal hepatic function General: Flushing, rare cases of hypersensitivity reactions, sometimes severe (eg, anaphylactic reactions, angioedema, bronchospasm, cardiopulmonary arrest, hypotension, laryngeal edema, laryngospasm, shock, shortness of breath, stridor) Local reactions: Pain, redness, and burning at injection site Lower respiratory: Hiccups Neurological: Oculogyric crisis, appearing alone, as well as with other dystonic reactions; transient dizziness during or shortly after intravenous infusion Skin and subcutaneous tissue: Urticaria, Stevens-Johnson syndrome, and toxic epidermal necrolysis Eye Disorders: Transient blindness (predominantly during IV administration) reported to resolve within a few minutes up to 48 hr; transient blurred vision Musculoskeletal and connective tissue: Arthralgia
Interaction
May reduce the analgesic effect of tramadol. Rifampicin and other CYP3A4 inducers may reduce levels/effects of ondansetron. Concomitant use of QT-prolonging agents (e.g. antiarrhythmics) may cause additive prolongation of QT interval. May increase the risk of arrhythmias w/ cardiotoxic drugs (e.g. anthracyclines). Potentially Fatal: May increase the hypotensive effect of apomorphine.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.